In the previous article, we have discussed the Gas constant in a very detailed manner, in which we have briefed about the Isothermal and Adiabatic Processes. In the Isothermal process, the temperature remains constant. Whereas the Adiabatic process, there is no heat exchange to and from the gas. Let us briefly discuss these to calculate pressure and the temperatures of the fluid under pressure. This is mostly used in Aeronautics, oceanography and metrology.

Pressure at a Point in Compressable Fluid
For compressible fluids, density (ρ) changes with the change in pressure and temperature. As we mentioned already these kinds of problems have huge scope in Aeronautics, oceanography and meteorology. Especially these problems help us when there is atmospheric air where density, pressure and temperature change with elevation.
Thus for fluids with variable density, the following Hydrostatic law equation discussed in the previous article cannot be integrated, unless the relationship between p and p is known.
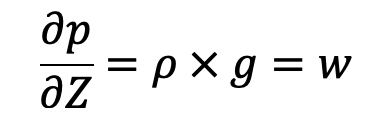
For gases the equation of state is
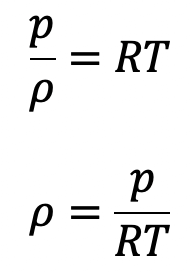
Now the above equation will be
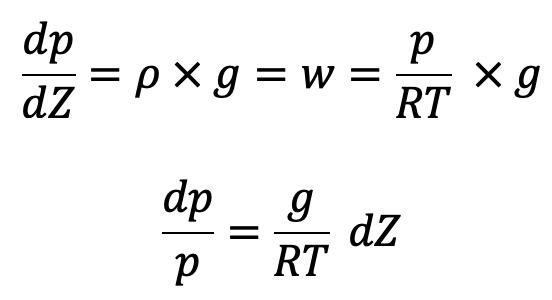
In the equation of Hydrostatic law, Z is measured vertically downward.
But if Z is measured vertically up, then
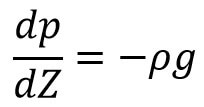
and hence the equation (b) will become
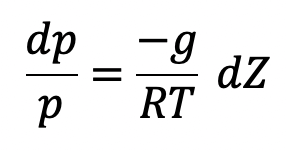
Isothermal Process
If temperature T is constant which is true for isothermal ess, equation (c) can be integrated as follows
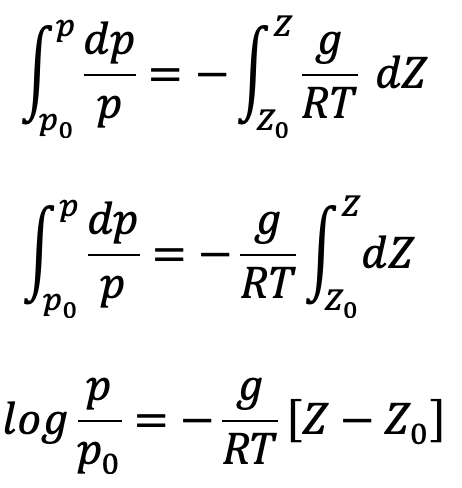
where p0 is the pressure where height is Z0. If the datum line is taken at Z0, then Z0 =0 and p0 becomes the pressure at the datum line.
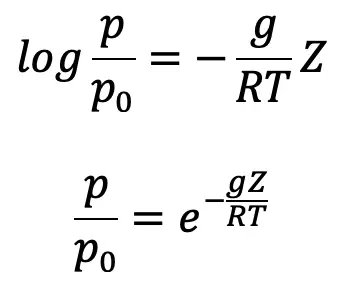
or pressure at a height Z is given by p = p0 e-gZ/RT …..(d)
Adiabatic Process
If temperature T is not constant but the process follows adiabatic law then the relation between pressure and density is given by
p/ρk Constant = C
where k is the ratio of constant.
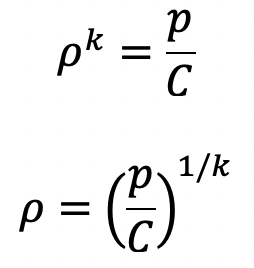
Then the equation of hydrostatic law for Z measured vertically up becomes,
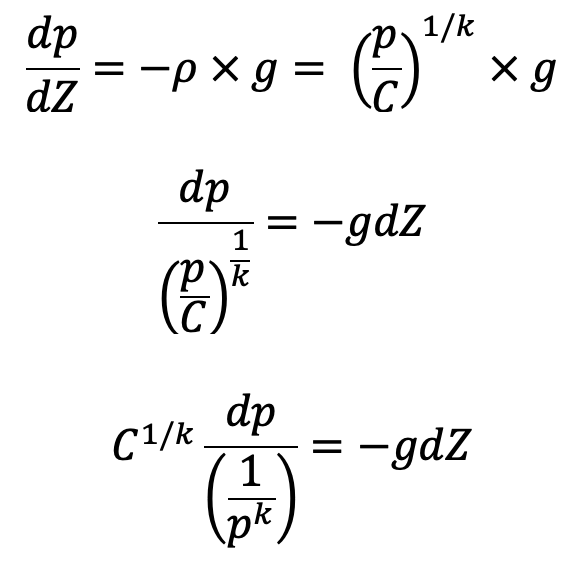
Integrating on both sides we get,
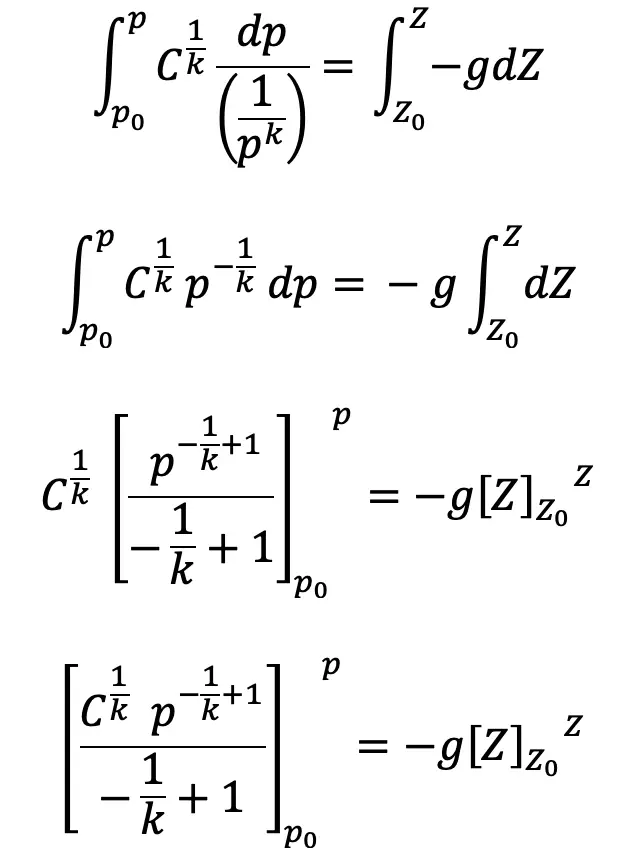
We have
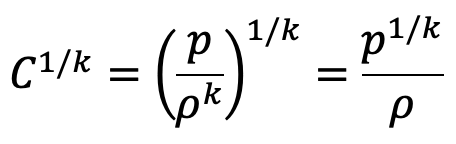
Substituting this value of C1/k above we get
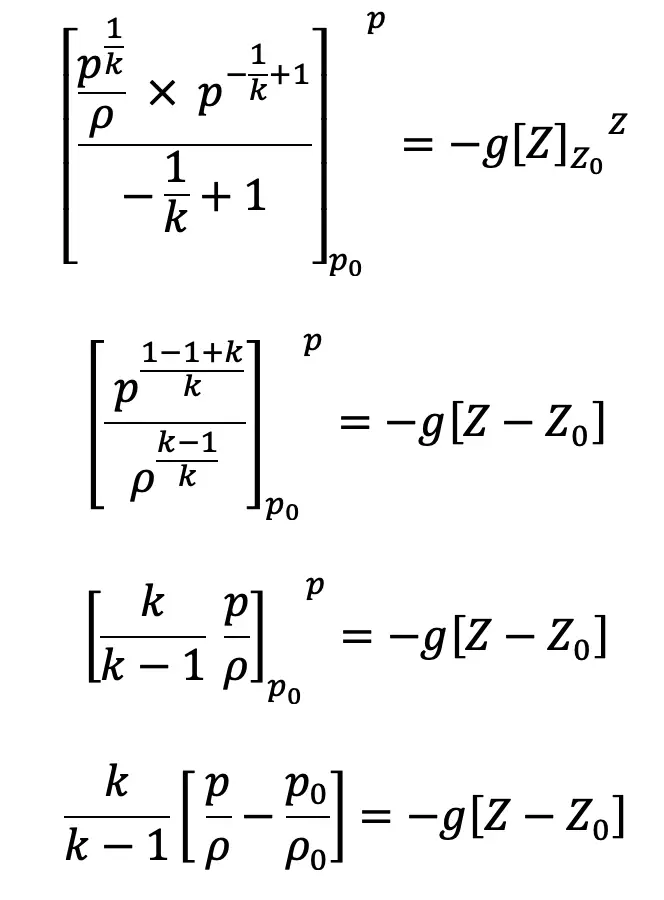
If the datum line is taken at Z0, where pressure, temperature and density are p0, T0, ρ0 then Z0 = 0
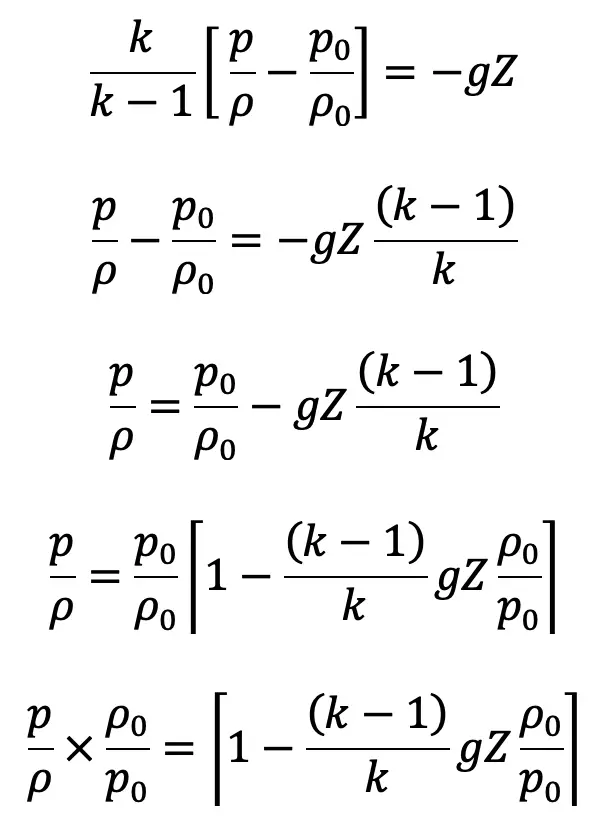
We know
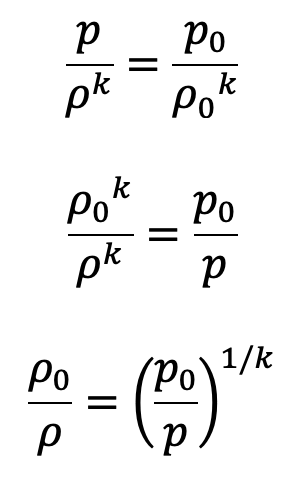
Substituting the value of ρ0/ρ in the above equation, we get
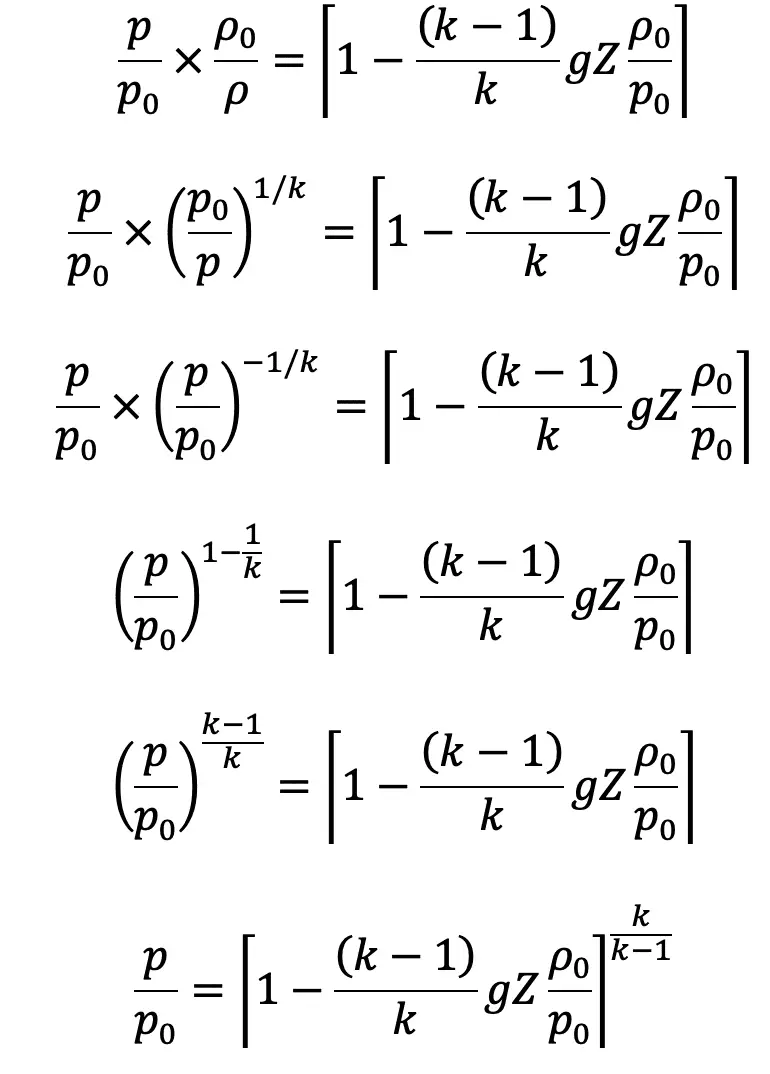
The pressure at height Z from the ground level is given by
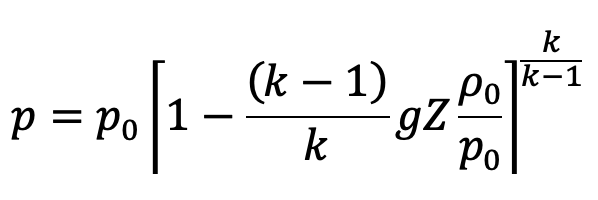
In the above equation (e)
p0 = Pressure at ground level, where Z0 = 0
ρ0 = Density of the air at ground level
The equation of state is
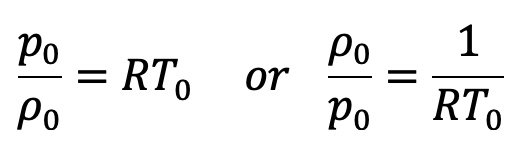
Substituting the values of ρ0/p0 in the above equation (e), we get
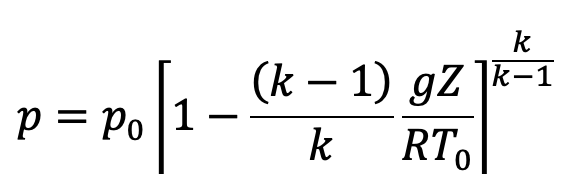
This is the equation to calculate the pressure at any point in the compressible fluid.
Temperature at any Point in Compressible Fluid
For the adiabatic process, the temperature at any height in the air is calculated similarly to the above derivation.
Equation Of State at ground level and at a height Z from ground level is written as
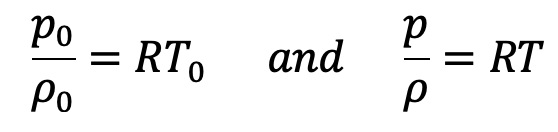
Dividing these equations we get
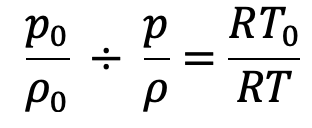
Further simplified and substituting the values we have from the above pressure equation (f), we get the following final derivation for the temperature.
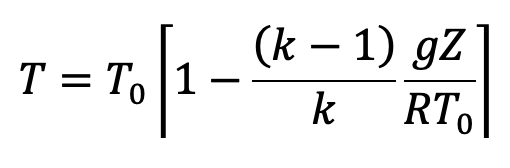
This is the equation to calculate the temperature at any point in the compressible fluid.
Temperature Lapse Rate (L)
It is defined as the rate at which the tem1Rrature changes with elevation. To obtain an expression for the temperature lapse rate, the temperature is given by equation (g) is differentiated with respect to Z as follows
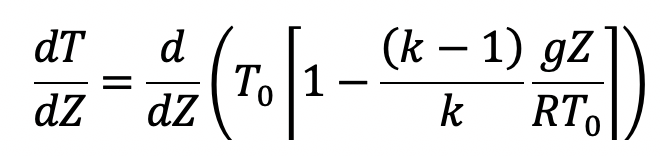
Where T0, K, g and R are constants.
The Temperature lapse Rate is denoted by L and hence
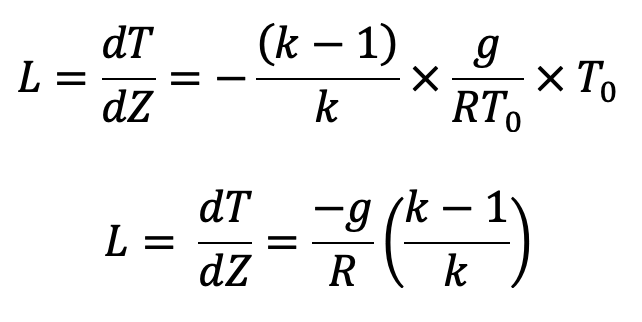
In the above equation (h),
(i) if k = I which means isothermal process, dT/dZ = 0, which means temperature dZ is constant with height.
(ii) If k > l, the lapse rate is negative which means temperature decreases with the increase in height.
In the atmosphere, the value of k varies with height hence the value of temperature lapse rate also varies.
From the sea level up to an elevation of about 11000m (or 11km), the temperature of the air decreases uniformly at the rate of 0.0065°C/m. from 11000m to 32000m, the temperature remains constant at -56.5°C and hence in this range lapse-rate is zero. The temperature rises again after 32000m m in the air.
Calculating Pressure on Sea Level
Let us solve an example problem to calculate the pressure of the atmosphere with different laws of fluids such as the isothermal adiabatic process.
Problem Statement: Calculate the pressure at a height of 7500 m above sea level if the atmospheric pressure is 10.143 N/cm2 and the temperature is 15°C at sea level. assuming
(i) Air is incompressible.
(ii) Pressure variation follows isothermal law
(iii) Pressure variation follows adiabatic law
(Take the density of air the sea level is equal to 1.285 kg/m3. Neglect variation of g with altitude.)
Solution:
Height above sea-level Z = 7500m
pressure at sea-level p0 = 10.143 N/cm2 = 10.143 × 104 N/m2
Temperature at sea-level t0 = 15°C; T0 = 273+15 = 288°K
Density of air, ρ = ρ0 1.285 kg/m3
(i) Pressure When air is incompressible
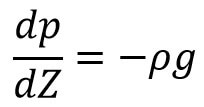
Intigrating the above relation on bothsides we get
p-p0 = – ρg[Z-Z0]
p = p0– ρgZ
p = 10.143 × 104– 1.258 × 9.81 × 7500
p = 101430-94543
p = 6887 N/m2
The pressure at a height of 7500 m above sea level if the atmospheric pressure is incompressible (p) = 0.6887 N/cm2
(ii) Pressure variation follows isothermal law
Using equation (d), we have
p = p0 e-gZ/RT
We have
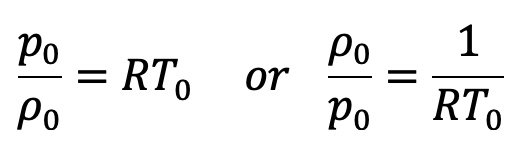
Substituting we get the following
p = p0 e-gZρ0/p0
p = 101430 × e -7500×1.285×9.81/101430
p = 101430 × e -0.9320
p = 101430 × 0.39376
p = 39939 N/m2
p = 3.993 N/cm2
The pressure at a height of 7500 m above sea level if the atmospheric pressure follows isothermal law is (p) = 3.993 N/cm2
(iii) Pressure variation follows adiabatic law
If the Pressure variation follows adiabatic law then k = 1.4
using the equation (e) we have
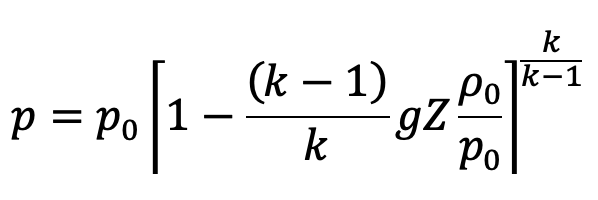
where ρ0 = 1.285kg/m3
Substituting all the values we have, we will get
p = 101430 [1-0.2662]3.5
p = 34310 N/m2
The pressure at a height of 7500 m above sea level if the atmospheric pressure follows adiabatic law is (p) = 3.431 N/cm2
This is how you can calculate the pressure on a sea level if you have the right inputs. Can you solve one more example problem on this topic…..?
Exercise Problem: If the atmospheric pressure at sea level is 10.143 N/cm2, determine the pressure at a height of 2500m assuming the pressure variation follows
(i) Pressure variation follows Hydrostatic Law
(ii) Pressure variation follows isothermal Law
(The density of air is given as 1.208 kg/m3)
Solve it and let me know the answers in the comment section below. All the best.

Leave a Reply