In the previous article, we have discussed the Carnot cycle and the Stirling Cycle with its efficiency. The Ericsson Cycle also resembles the Carnot cycle with the constant pressure process and the constant temperature processes. Whereas the Stirling cycle is a constant volume with a constant temperature.
Ericsson Cycle
In this Ericsson Cycle, there are two Isothermal processes and two Isobaric processes (constant Pressure processes).
The P-V diagram and T-S diagrams for the Ericsson Cycle shown below.
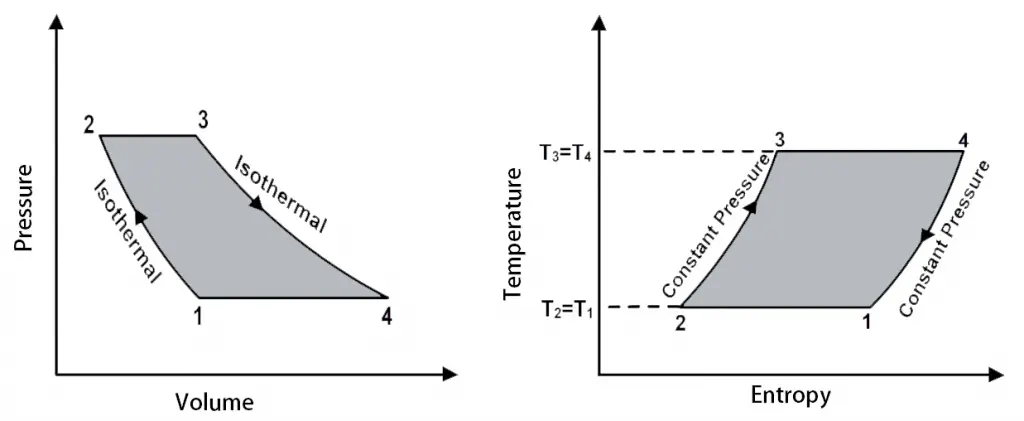
P-V diagram and T-S diagrams for the Ericsson Cycle
The different processes in the Ericsson cycle.
- 3 → 4 (Isothermal Expansion)
- 4 → 1 (Isobaric Expansion)
- 1 → 2 (Isothermal Compression)
- 2 → 3 (Isobaric Compression)
The only difference between the Stirling cycle and the Ericsson Cycle is that, during the processes 4 → 1 and the 2 → 3, instead of the constant volume processes, they have made it as the constant pressure processes (Isobaric).
The main advantage of the Ericsson cycle over the Carnot cycle and the Stirling Cycle is, it maintains the smaller pressure ratio for the given ratio of maximum to minimum specific volume with the higher Mean Effective pressure.
In this thermodynamic cycle, the heat addition and the heat rejection take place in both the Isobaric (Constant Pressure) and the Isothermal Processes.
The net effect is that the heat needs to be added only at constant temperature T3 = T4 and rejected at the constant temperature T2 = T1
The efficiency of the Ericsson Cycle is
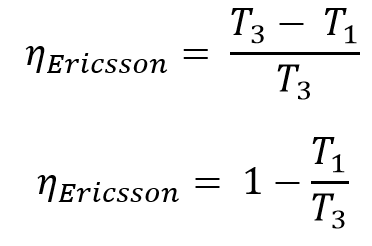
Same as the Carnot Cycle
Conclusion
This Ericsson Cycle is used in the gas turbine engines by introducing a large number of stages with the help of heat exchangers insulators, reheaters. This cycle is not applicable to build the piston engines. If you have any thoughts about this topic, leave comments below.

Leave a Reply