Dual cycle in thermodynamics is a mixed cycle from the Otto cycle and the diesel cycle. We have already discussed these cycles with the P-V and T-S diagrams in the previous articles. In this article, we are going to discuss the Dual cycle with the P-V diagram and the T-S diagram.
Dual Cycle
The Otto cycle is a constant volume cycle and the Diesel cycle is a constant pressure cycle. In a practical scenario, in the constant volume cycle, the combustion might not take place at the constant volume because of the chemical reaction during the combustion need some time to take place.
Similarly, in the Constant pressure cycle, the combustion might not take place at the constant pressure because of the uncontrolled rapid combustion. So this is the difference between the air standard cycles and the practical engines.
This Dual cycle is a compromise between the Otto cycle and the Diesel Cycle. so it is called the mixed cycle or Limited pressure Cycle.
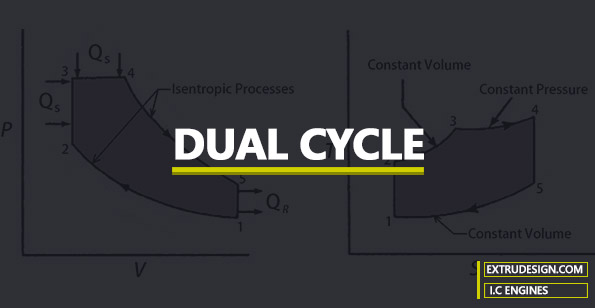
The P-V and T-S diagram for the Dual cycle is shown below.
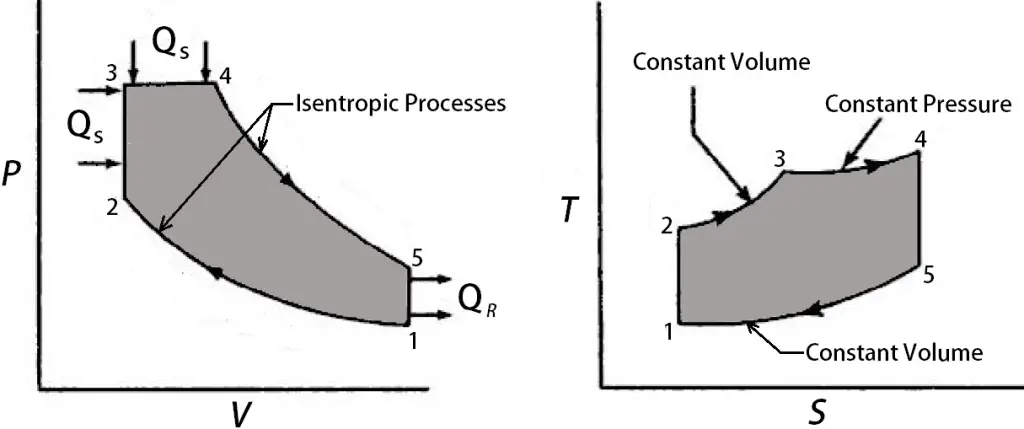
0 → 1 and 1 → 0
When the engine is working on the full throttle, the Process 0 → 1 and 1 → 0 represents the suction and the exhaust process of Thermodynamic cycle in the P-V diagram and the effect of these two processes considered as nullified.
1 → 2
The process 1 → 2 is the isotropic compression of the air in the cylinder while piston moves from the bottom dead centre (BDC) to the top dead centre (TDC)
2 → 3 & 3 → 4
During the process, 2 → 3 First the heat is supplied at a constant volume and then the remaining part of heat will be added at the constant pressure process 3 → 4.
4 → 5 & 5 → 1
These two processes 4 → 5 & 5 → 1 will represent the isotropic expansion (Piston moves from the Top dead centre to the bottom dead centre) and the constant volume heat rejection respectively.
Thermal Efficiency
Thermodynamically, the efficiency of the Dual cycle is given by
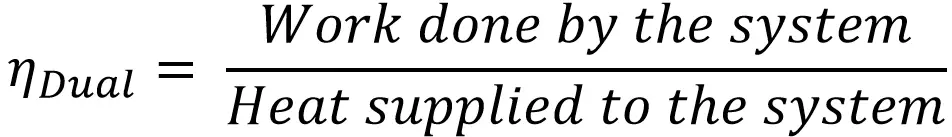
Work done by the system = Heat supplied (QS)- Heat rejected (QR)
W = QS – QR
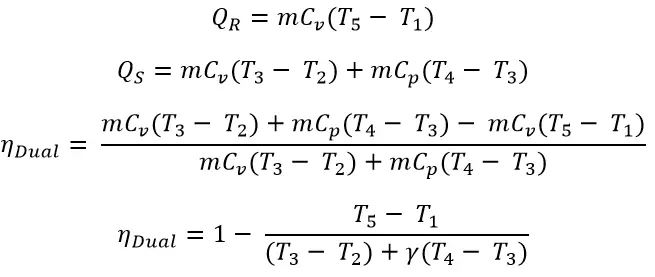
From the processes 1 → 2
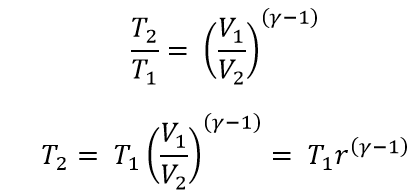
Where r is the Compression Ratio
Now from the process 2 → 3 constant Volume process
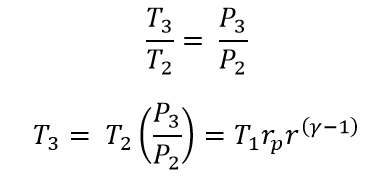
(Substituted the T2 in the above equation)
Where rp is the pressure ratio in the constant volume process which is equal to the P3/P2
From the 3 → 4 Constant pressure process
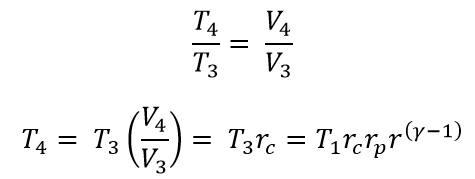
(Substituted the T3 & T2 in the above equation)
Where rcrc is the Cut-Off ratio in the constant pressure process which is equal to the V4/V3
From the Isotropic process 4 → 5
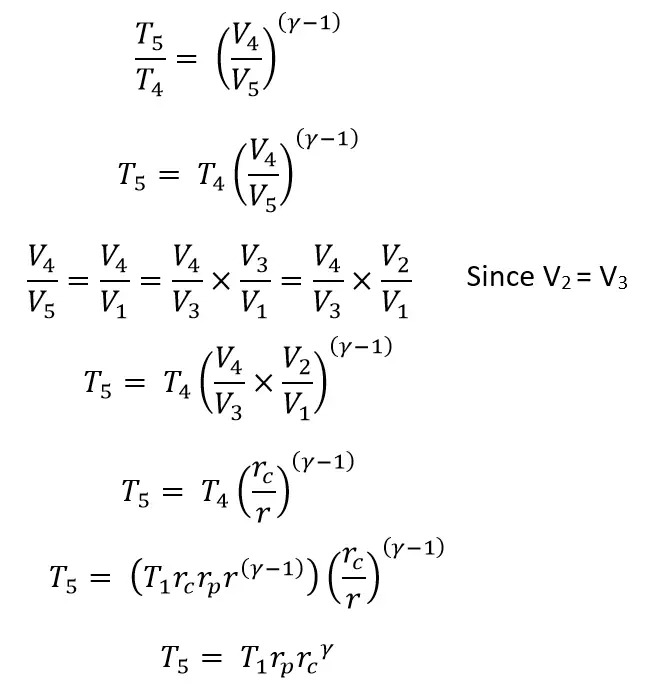
(Substituted the T4 & T3 & T2 in the above equation)
Let’s substitute all these above equations in the main efficiency equation
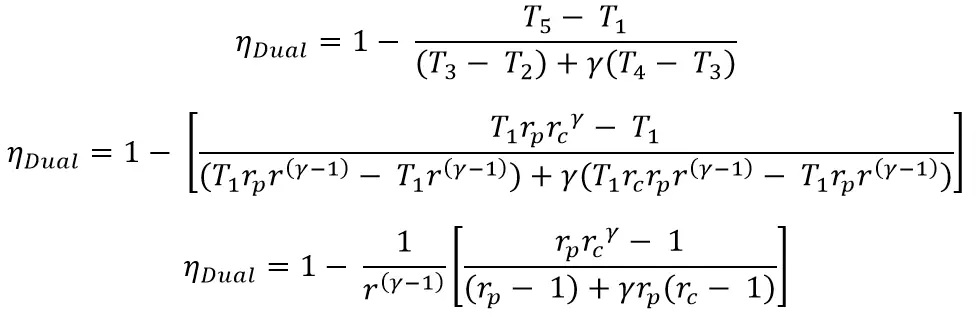
From the above equation, we can observe that the value of rp>1 results in increased efficiency for the given value of rc and γ.
Where rc = 1 then it will give the Otto cycle efficiency and with the rp = 1 it will gives the diesel cycle efficiency.
Conclusion
We have discussed the mixed cycle called Dual Cycle with the P-V and T-S diagrams and also we have derived an equation for the Efficiency of the Dual Cycle. Please let us know your thought in the comment section below.

Nice