The galvanizing process is a specific coating of zinc on on metals to prevent rusting. Whereas electroplating is various options of metal for coating for many other reasons. Let us discuss these Galvanizing and Electroplating Processes with neat schematic diagrams.
Galvanizing Process
Galvanizing is the name given to the process of coating steel sheets with a layer of zinc. The layer of zinc protects the steel item from corrosion and rusting by sacrificing itself. Over the years, when all zinc has been depleted due to atmospheric action, the steel surface will get exposed and begin to rust. Galvanised metal sheets, pipes, and wires are used very extensively as the process of galvanizing is a low-cost process, but adds a lot of value to the item.
The main techniques for galvanizing are:
- Hot-dip galvanizing
- Cold dip galvanizing
- By electroplating
1. Hot-dip Galvanizing
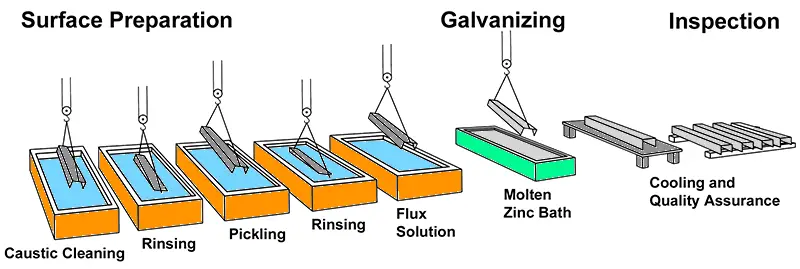
The part to be galvanized is cleaned thoroughly. The idea is to remove all grease, paint, rust, dirt, etc. from the surface. If a steel sheet is to be galvanized, the sheet is annealed and cooled in an oxygen-free atmosphere. When it has cooled sufficiently, it is dipped into a bath of molten zinc. The sheet is then drawn through two rollers when all excess zinc is removed from the surface and the zinc layer becomes uniform in thickness.
2. Cold Dip Galvanizing
Cold dip galvanizing process is cheaper but more time-consuming. The first step is a thorough cleaning of the surface as in hot-dip galvanizing. Then the part or sheet to be galvanized is made to hang in a cold zinc bath consisting of zinc chloride, tin chloride, and some other salts. The parts remain dipped or suspended in a cold bath for 3 to 12 hours depending upon the thickness of coating required. Bath, however, has to be periodically stirred.
The process requires no power for heating the bath. Besides zinc has a tendency to sublimate and in the hot-dip process, a lot of zinc gets wasted due to sublimation. Hence cold dip process proves ultimately cheaper and better.
3. By Electroplating
This process is used only for depositing a layer of zinc on intricately shaped items. It is not a popular process for mass production. The basic principle of zinc electroplating is to use an electrolyte made by dissolving zinc chloride, zinc sulfate, ammonium chloride, and ammonium sulfate in distilled water. The zinc metal is used as anode and the article to be plated is used as cathode. Upon using a suitable low voltage and direct current, zinc gets deposited on the cathode.
Electroplating Process
Working Principle of Electroplating Process
In the process of electroplating, a thin layer of a metal is deposited on another metal part with the object of corrosion prevention, or to ensure that the electroplated part looks nice and aesthetic. The part is usually electroplated with gold, silver, chromium, or nickel because these platings look nice and do not tarnish.
The principle of electroplating is simple. Suppose two electrodes are immersed partially in a suitable electrolyte and a direct current is passed by joining the two electrodes in an external circuit. In that case, the metal from the anode gets transferred through electrolyte action on the cathode. A simple arrangement for electroplating is shown in the following figure.
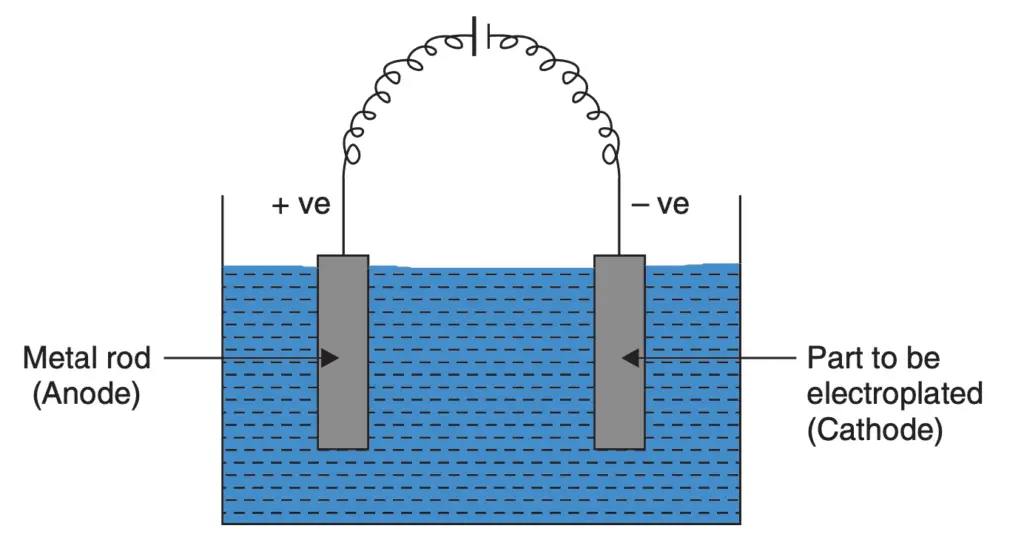
Electroplating is a metal deposition process. The amount of metal deposited can be calculated easily, it is remembered that 96500 coulombs of electrical charge (1 coulomb = 1 amp current × 1 second) deposits one electrochemical equivalent of the substance at the electrode, irrespective of what the substance is.
The electrochemical equivalent of a substance is equal = Atomic weight of the substance (gms)/ Valency
For example, if copper is being deposited from CuSO4 solution, the electrochemical equivalent of copper is the atomic weight of copper ÷ 2 (valency of copper).
The atomic weight of copper is 63.5, and its equivalent will be = 31.75 gms.
Faraday’s Laws Of Electrolysis
The process of electrolysis or electroplating is governed by two laws called Faraday’s laws of electrolysis. These laws are:
- First law: The amount of substance liberated (or deposited) at an electrode during electrolysis is directly proportional to the total electrical charge passed in the electrolyte.
- Second law: When the same quantity of electric charge is passed through different electrolytes connected in series, the masses of substances liberated (or deposited) at the electrodes are directly proportional to their electrochemical equivalent weights.
The net result of these two laws is that 96500 coulombs of electric charge will release or deposit one electrochemical equivalent in grams (called one gram equivalent) of the substance concerned.
A numerical will make this clear.
Example Problem: An electric current is passed through two cells connected in series and containing CuSO4 and AgNO3 solutions. The masses of copper and silver deposited are 0.424 gm and 1.44 gms respectively. Find the equivalent mass of silver, if that of copper is 31.75.
Answer:
According to Faraday’s second law, (since cells are connected in series)
1.44 : 0.424 = equivalent mass of silver/equivalent mass of copper
Equivalent mass of silver = 1.44/0.424 × 31 75
Equivalent mass of silver = 107.8
Conclusion
We have discussed about the different Galvanizing process. Electroplating is one of the Galvanizing process and discussed Faraday’s Laws Of Electrolysis with an example problem. Let us know what do you think about this article in the comment section below.

Leave a Reply